


The promise of digital transformation and biopharma 4.0 is exciting, but even the most advanced ML/AI applications are useless if the source data hasn’t been properly curated. Yet the problem with these point solutions is that they don’t address the core challenge: capturing context-rich data at the point of entry with the right metadata and structure to enable searching, reporting, and analytics across the development lifecycle. There is a plethora of software applications designed to tackle different aspects of product and process development, from design of experiments (DoE) through instrument integration/automation and data aggregation/visualization.
So why, when clones can now be grown and screened on a chip and digital twins can apply the power of AI to predict and increase process performance, is this still the industry’s Achilles heel? In addition, 62% of participants reported spending at least five hours a week on data administration and in some cases more than 20 hours a week. In a recent Aspen survey, 50% of the participants were using legacy applications such as electronic lab notebooks (ELNs) to record process development work and the other 50% were using a mix of paper/Excel and standalone instrument software. What many people outside of the industry don’t realize and would be surprised to know is that the data backbone that ties together all the activities from research and discovery through process development and manufacturing is still mainly Microsoft Office documents. Many end up failing at the last hurdle, clinical trials, after millions of dollars have already been spent.
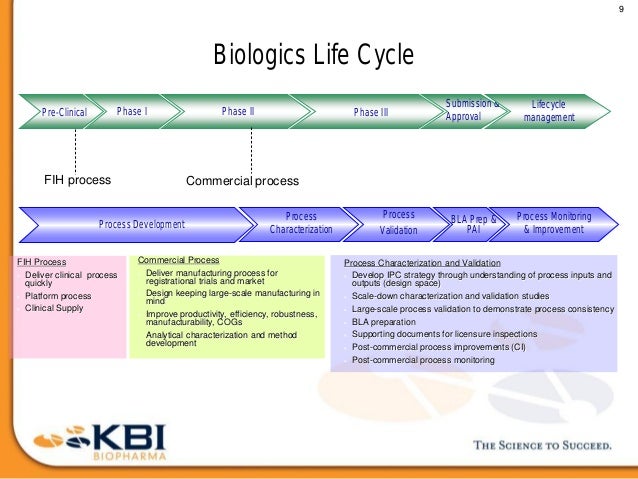
This has long been a murky topic and is usually discussed in terms of risk: biologics are highly complex both in terms of their structure and mode of action and for every successful product that makes it to market, there is a long trail of unsuccessful candidates. It’s also raising questions about why it typically takes so long and costs so much to bring these products to market. The global pandemic is shining a spotlight on the power of biologics, both vaccines and antibody therapeutics, to prevent serious infections and treat disease.


 0 kommentar(er)
0 kommentar(er)
